We compared the shape of the curves given by the two models. Solving the continuous
model here is beyond the scope of this module (that would get into a lot of
calculus), but we can do the discrete model with a modest amount of algebra.
Let's go back to sugar water, with the following assumptions:
- Initial concentration on left = 1 M
- Initial concentration on right = 0 M
- P = 0.1 cm/sec
- A = 1 cm2
- equal volumes on both sides of membrane
We want to know what the final concentrations will be over time , i.e., at time 1 sec, 2 sec, 3 sec, 4 sec....... So lets set up our equation from above we know that :
Δn/Δt = 0.1 cm/sec x 1 cm2 x (1M - 0M) = 0.1 moles/sec
So in the first second, approximately 0.1 moles change sides (from the more concentrated to the less concentrated side). That leaves
on the left side: 1 moles - 0.1 moles= 0.9 moles/liter
on the right side: 0 moles + 0.1 moles= 0.1 moles/liter
How about the concentrations at time 2 seconds? We need to readjust our estimate of diffusion rate by plugging in the new concentrations. Everything else stays the same:
Δn/Δt = .1 cm/sec x 1 cm2 x (.9M - .1M) = .08 moles/sec
and the new concentrations are 0.82 and 0.18M.
on the left side: 0.9 moles - 0.08 moles= 0.82 moles/liter
on the right side: 0.1 moles + 0.08 moles= 0.18 moles/liter
Continuing on like this,
Δn/Δt at 3 seconds = 0.074 moles/sec,
C on left = 0.746 M, C on right = 0.254 MΔn/Δt t at 4 seconds = 0.049 moles/sec,
C on left = 0.697 M, C on right = 0.303 MΔn/Δt t at 5 seconds = 0.039 moles/sec,
C on left = 0.658 M, C on right = 0.342 MΔn/Δt at 6 seconds = 0.032 moles/sec,
C on left = 0.626 M, C on right = 0.374M
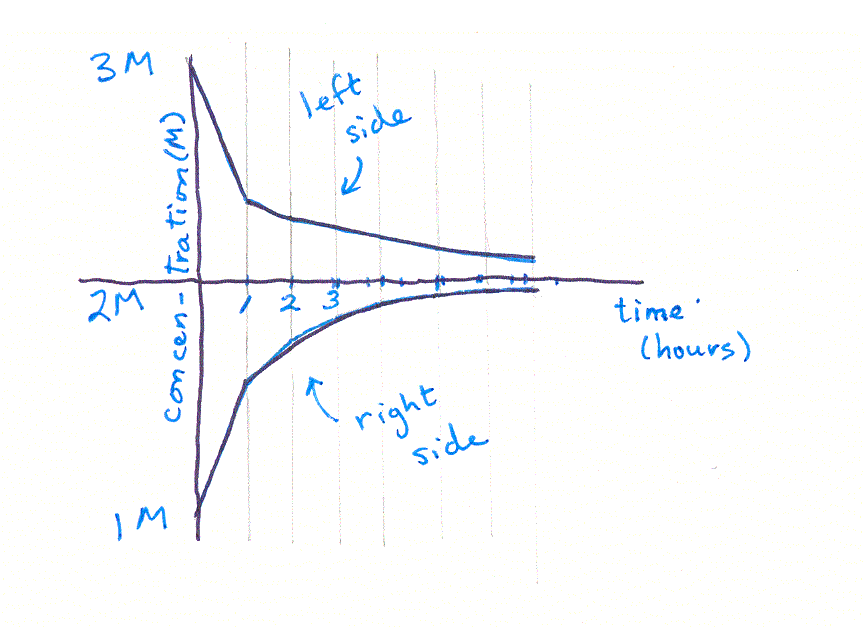
This process is called "iteration" -- you iterate, or repeat, the equation over and over, each time substituting in the values calculated in the last iteration. You can see that, if we continued iterating long enough, we would eventually reach a point where the two concentrations were equal -- in other words, an equilibrium. (It would take a long time though, better use a spreadsheet rather than a calculator!) If you graph the two sets of concentrations, you'll see something like this:
Congratulations, you've reached the end of the diffusion through a membrane section! You can now proceed to take the quiz...
If you want a printer-friendly version of this module, you can find it here in a Microsoft Word document. This printer-friendly version should be used only to review, as it does not contain any of the interactive material, and only a skeletal version of problems solved in the module.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu