Quick refresh: molecular weight
A quick refresher on molecular weight: if you look at a periodic table, every element has a number and a molecular weight. The molecular number tells you how many proton an atom has, and the molecular weight gives you the average weight of an atom (including protons and a range of possible numbers of neutrons). So, no matter how your periodic table is organized, the molecular wieght is the larger of the numbers listed in the element's box:
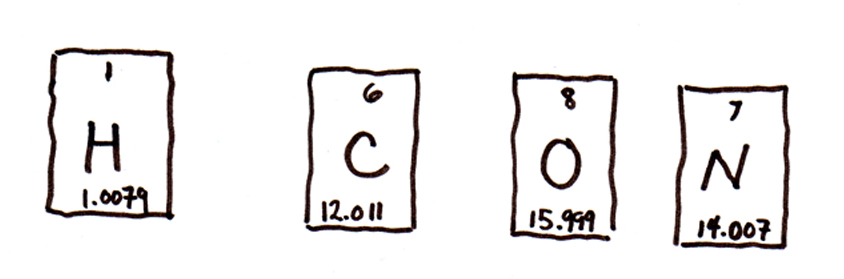
|
|
|---|---|
| blurry table of elements | some excerpts |
By definition, one mole of an element weighs the same
(in grams) as the molecular weight.
For those of you who are thinking, hold on, what's a mole ... a mole is a
more convenient way to count up molecule. As everyone knows, your average
fist-sized object contains a billion-gazillion molecules, which makes them
rather inconvenient to count. So instead, we talk about a
"mole" of molecules, which means 6.022 * 1023, or about
60 trillion trillion. Kind of like a "dozen" eggs, but a
much bigger number. 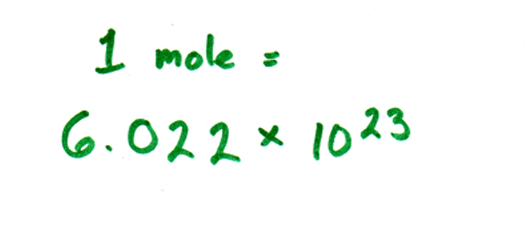
So anyway, one mole of carbon is the same as 60 trillion trillion carbon atoms and weighs all of 12 grams. Likewise, a mole of hydrogen weighs a paltry 1 gram, and a mole of oxygen tops out at 16 grams. (We're rounding a little here, which is no big deal).
To get a single glucose molecule (C6H12O6), we need 6 carbon atoms, 12 hydrogen atoms, and finally 6 oxygen atoms. Likewise, to get a mole of glucose, we need 6 moles of carbon, 12 moles of hydrogen, and 6 moles of oxygen. All of this weighs
6*12 + 12*1 + 1*16 = 180 grams.
So, the molecular weight (or weight of a mole) of sugar is 180g.
The chemical formula of caffeine is C8H10N4O2. Using the periodic table excerpts from below, what is its molecular weight?

(To make this problem interactive, turn on javascript!)
- I need a hint ... : A caffeince molecule has 8 carbon atoms and each
weights about 12 g/mol.
- ...another hint ... : Add up the weight of each atom type separately.
I think I have the answer: 8 * 12 + 10 * 1 + 4 * 14
+ 2 * 16
= 166 g/mol
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu