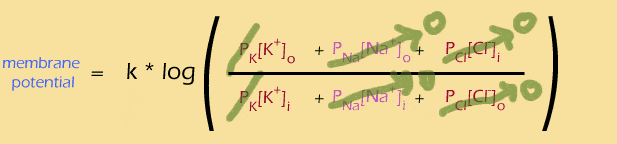The Nernst Equation
Remember what happened when we specified that the membrane should only be permeable to ONE ion (in this case K+):
Here is an even simpler way of stating the same thing, called the "Nernst Equation", and as an added bonus I'll even let you in on the value of the mysterious "k": at normal body temperature, 37°C, “k” is equal to 62 if you measure potential in millivolts AND if you use logarithms in base 10.
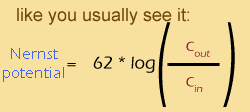
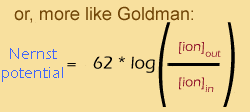
This equation was actually discovered half a century before the Goldman equation, by a Nobel prize winner named Walther Nernst -- hence, the Nernst equation. In the standard version of the equation "C" stands for "concentration". I included the second version just to make the relationship between the Nernst and the Goldman equations more apparent. As you can see The Nernst equation expresses the membrane potential for one particular ion when it is at chemical equilibrium, whereas the Goldman equation takes into account all ions.
all ions.
By the way, the value of k = 62 mV comes from the calculation of 2.303 x RT/ZF, where 2.303 is the conversion from log base e to log base 10, R is the constant from the gas equation, T is temperature in Kelvin (the absolute temperature), F is the Faraday constant and Z is the valence of the ion. Hence the value of 62 is valid only at body temperature (37 degrees Celsius).A deep sea fish would have a different value, because of the lower effect of temperature on the potential energy of the ions!
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu