Going with the flow
For most of this module, we'll focus more on channels than on pumps. It's not that pumps aren't important -- in fact, as we'll discuss later, they "set the stage" for everything that's going to happen. Once the stage is set, channels become the interesting part of the system.
An open channel allows conditions inside and outside of the cell to affect each other. And there are two major differences between the inside and the outside:
- The concentrations of ions are different inside vs. outside the cell. We'll be creative and call this "C" for concentration: in fact, we have a CONCENTRATION GRADIENT which can power diffusion. Furthermore, each ion has ITS OWN concentration gradient. In other words, K+ ions inside the cell only "care" about how many K+ there are on the outside -- they don't give a fig about Na+ or Cl- or any other anions (A-).
- As positive (+) ions move through the cell membrane, they can't take negative (-) ions with them, and this results in a slight charge imbalance, inside vs. outside the cell. We'll call this a VOLTAGE GRADIENT, or just V. Charged particles "want" to move down a voltage gradient, just like dissolved particles "want" to move down a concentration gradient.
Since ions are both charged and dissolved particles, they have two different gradients to worry about, a chemical gradient and an electrical (voltage) gradient!
Why do I say "two different gradients"? Because the concentration gradient and the voltage gradient don't necessarily have the same effect on the particle -- in fact, these two gradients are often OPPOSED to each other.
If a ball is on two opposed gradients, it will come to rest in the middle, right?
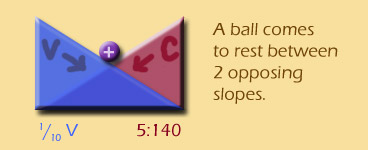
Well, if ions are being affected by two opposing gradients, they will also come to an equilibrium position. Once that equilibrium is found, the ions can still move back and forth, but the NET movement will be zero -- the definition of an equilibrium.
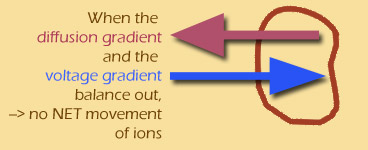
(Throughout this module, I'll use electric blue to show the voltage gradient, and plum red for the diffusion gradient.)
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu