Meet the neighbourhood
So what's inside (and outside) a typical cell? These are approximate numbers only. We're talking about any cell in your body at the moment, whether it's in your fingertip, femur, or islet of Langerhans:
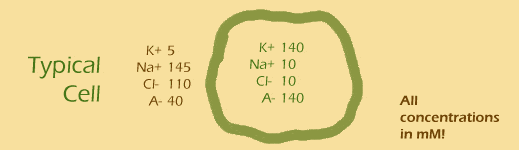
(A- here stands for other anions).
Let's try adding up the charges on the inside and outside (and remember that Cl-and other unspecified anions (A-) have negative charges, so these numbers get subtracted!):
Outside: 5 + 145 - 110 - 40 =
Inside: 140 + 10 - 10 - 140 =
Hmm, the inside and the outside of the cell both have total charge of 0 That's probably a good thing, since you wouldn't really want your cells to be charged.
charge inside cell = charge outside cell = 0 !!
However, it does raise a question: if the charge on both sides is zero, why should there be a voltage gradient??? The key is that the membrane is only permeable to SOME of the ions (usually positively charged ones). Namely, for most cells most of the time, channels are open for potassium (K+) but closed for other ions. (Actually by "open", we mean that individual channels pop open randomly for a few milliseconds at a time, like the video on the right.)
As I said a few screens back, when K+ leaves the cell by itself, it creates a small imbalance in charge. But this imbalance is SOOOOO small that we couldn't measure it in a useful way. If I wanted to include it on the diagram above, I would need to say something like
[K+] inside = 139.9999999999mM
[K+] outside = 5.0000000001mM
Amazingly, this slight difference in concentrations is enough to cause a pretty significant voltage gradient. You can measure this electrical potential difference by placing electrodes inside and outside of the cell. When we do this, there is a small electrical potential difference, with the inside of the cell being negative.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu