Our equations are ready
You will recall from the diffusion module that flux is equal to the flow multiplied by the area (A) through which the substance is flowing. If we multiply the flux equations by A and use our new expression for the permeability P, in place of D, we can express the flow as:
| Continuous version |
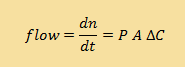
|
|
|---|---|---|
| Discrete version |
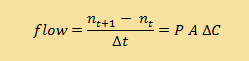 |
We can also express flux (flow rate divided by the area) in terms of the permeability and the difference in concentration between both sides:
flux = P ΔC
In the case of diffusion through a membrane then, flux is equal to the permeability multiplied by the difference in concentrations on either side. In order to do any calculations, we will have to get a handle on the actual values of these variables.
That means we need to be able measure concentration. You might think this
is pretty easy. For example, if you have 100 grams of sugar dissolved in a total volume of 1L of water, you will have a solution that is 10% sugar by weight, and you probably
don't want to try to drink it. (Honest, even Red Bull is only about 9%
by weight). 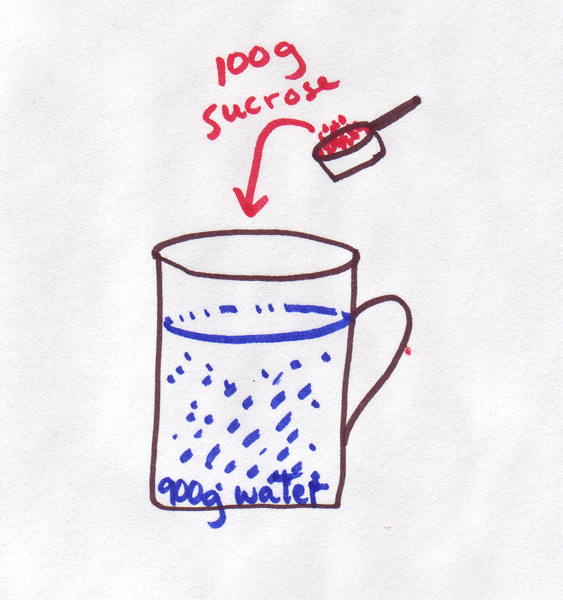
However, unfortunately, diffusion doesn't care about weight, it cares (if a process can be said to care) about particles. How many particles of sugar were in that 100 grams?
Before you get out a magnifying glass and start counting sugar grains, let's be clear about what a particle is: as a first approximation, a particle is a molecule. So, you need to know the molecular weight of sugar C12H22O11, which is 342g. Thus 100g of sugar is 0.29 moles and the concentration is 0.29 M.
If you need a refresher on how to calculate molecular weight and molarity, you should revise the module on this topic.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu