Simplifying the situation
But first, back to biology. We're going to talk about what happens when you have a concentrated solution (think sugar water) and a less concentrated solution (think distilled water). Here are the equations again:
| Continuous form | 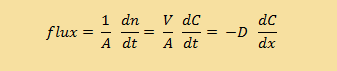
|
|---|
| Discrete form | 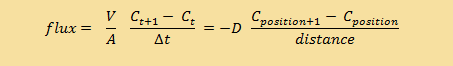
|
|---|
Remember that we were talking about the diffusion happening in a sort of imaginary tube, like this:
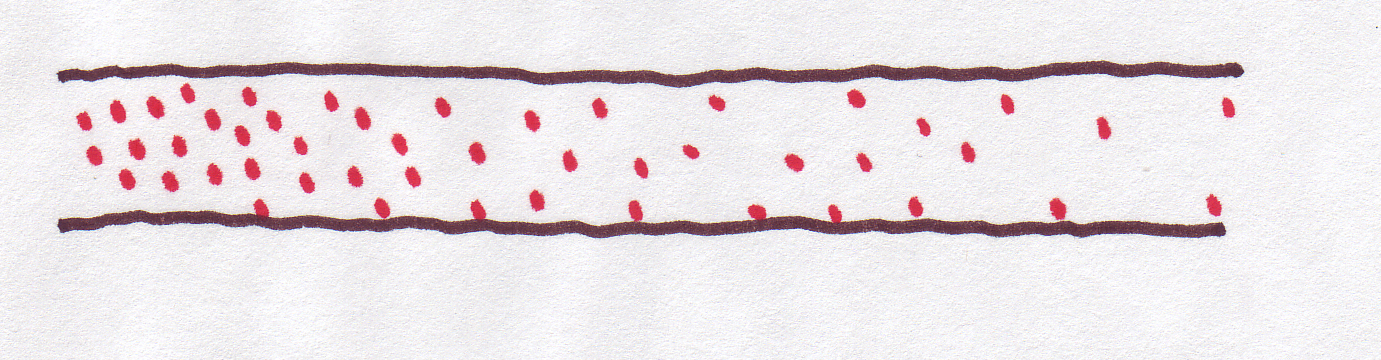
That's why we talked about concentration at "positions" along the tube. But
in this module, we simplify the situation: just two sides of membrane -- inside
and outside, or left and right. (Or the right side and the wrong side of the
tracks, or however else you want to think about it). Politics aside, right
and left is a lot easier to deal with than a spectrum of possible positions.
| Two-Compartment Model | 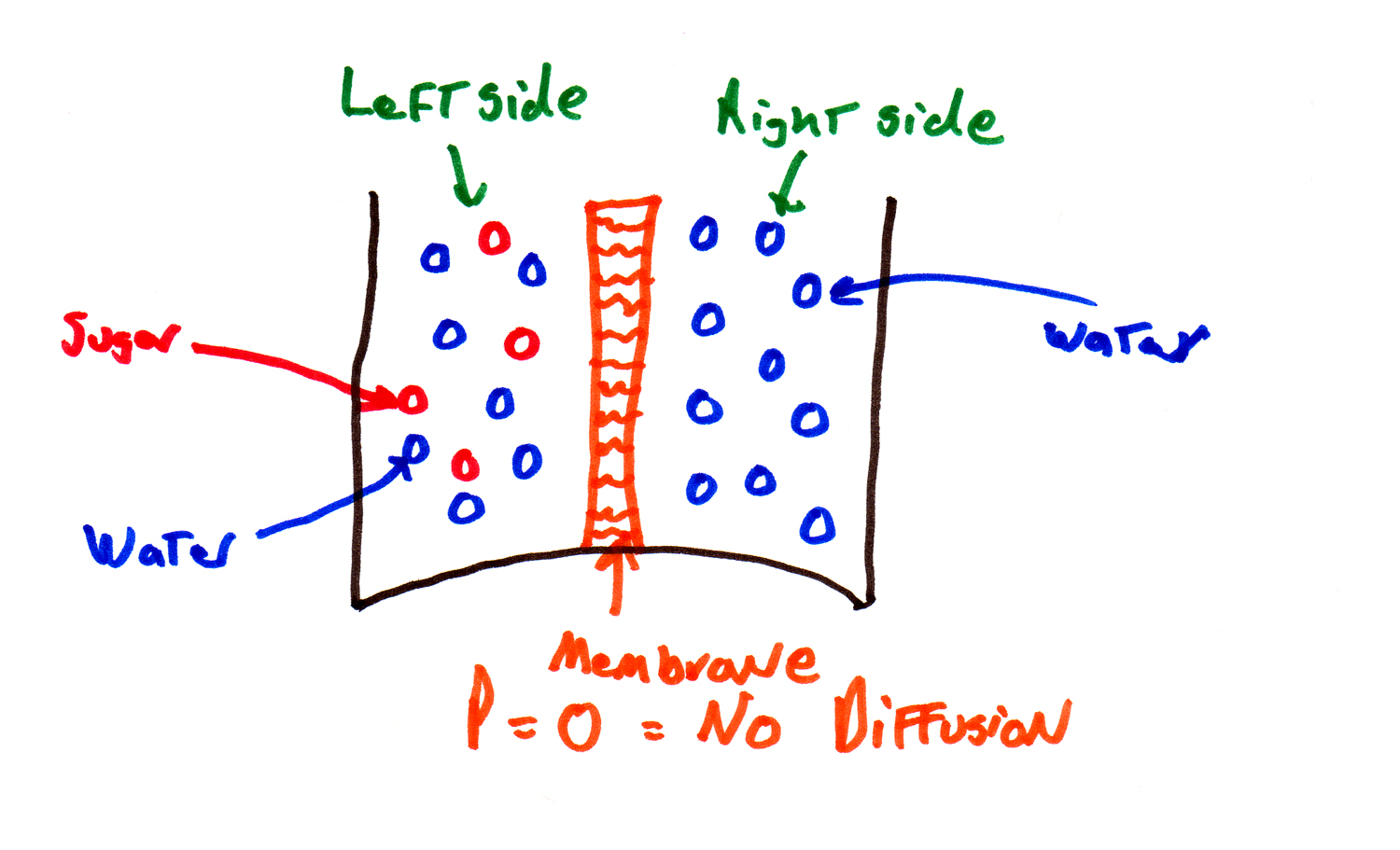
|
|---|
Here are a couple of other assumptions we need to make about the state of the system (think of this as the legal fine print):
1. The solutions on both sides of the membrane are homogeneous. By this we mean that the solute is evenly mixed so there is no active diffusion taking place within a side.
2. The membrane is not changing width, so the distance between the sides is a constant.
This is called the "two compartment model" -- the continuous positions in the original equations are replaced by two compartments, and this is going to make the equations easier to deal with.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu