Meet the neighborhood
So what's inside (and outside) a typical cell? These are approximate numbers only. We're talking about just about any cell in your body at the moment, whether its your fingertip, femur, or islet of Langerhans:
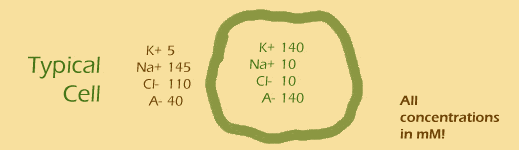
Let's try adding up the charges on the inside and outside:
Outside: 5 + 145 - 110 - 40 =
Inside: 140 + 10 - 10 - 140 =
Hmm, the inside and the outside of the cell both have approximately 0 charge. That's probably a good thing, since you wouldn't really want your cells to be charged.
charge inside cell = charge outside cell = 0 !!
However, it does raise a question: if the charge on both sides is zero, why should there be a voltage gradient??? The key is that the membrane is only permeable to SOME of the ions (usually positively charged ones). Namely, for most cells most of the time, channels are open for potassium (K+) but closed for other ions. (And actually by "open", we mean that individual channels pop open randomly for a few milliseconds at a time), like the video on the right:
So, as I said a few screens back, when K+ leaves the cell by itself, it creates a small imbalance in charge. But this imbalance is SOOOOO small that we couldn't measure it in a useful way. If I wanted to include on the diagram above, I would need to say something like
[K+] inside = 139.9999999999mM
[K+] outside = 5.0000000001mM
Amazingly, this slight difference is enough to cause a pretty significant voltage gradient.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu