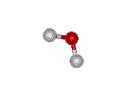
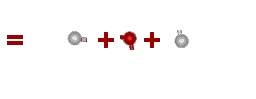What about more complicated molecules?
How about a mole of water? You know that the formula for water is H2O. So, if you broke all the bonds, a mole of water would actually contain TWO moles of hydrogen and ONE mole of oxygen.
|
|
 You can then use the periodic table to determine how much each of those weighs:
You can then use the periodic table to determine how much each of those weighs:
total weight = 2 x Hydrogen + 1 x Oxygen
total weight = 2 x 1.008g + 1 x 15.99g
total weight = 18.006g
So a mol of water is about one swallow.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu