Molar weights made easy
The guy who came up with 602,200,000,000,000,000,000,000 (named Avogadro) probably didn't like all those zero's any more than you do. So he did something very smart: he defined a mol as
the number of carbon atoms in 12 grams
It turns out 602,200,000,000,000,000,000,000 is approximately that number.
 But why
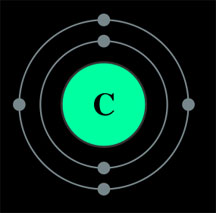
12 grams? Well, if you remember something about atomic structure, you know that a carbon atom has six
protons and six neutrons (which means it has 6 electrons as well, 4 of which are on its
outer shell, allowing it to form 4 covalent bonds and be backbone of organic
life ... but that's a different story)
But why
12 grams? Well, if you remember something about atomic structure, you know that a carbon atom has six
protons and six neutrons (which means it has 6 electrons as well, 4 of which are on its
outer shell, allowing it to form 4 covalent bonds and be backbone of organic
life ... but that's a different story)
Anyway, back to the 12 grams. Since a proton and a neutron each weigh 1 atomic unit, a carbon atom weighs about 12 atomic units (the electrons don't weigh much of anything). And a mol of carbon weighs 12 grams. That means 1 mol of protons or neutrons weighs 1 gram.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu