Action Potential and the Goldman Equation
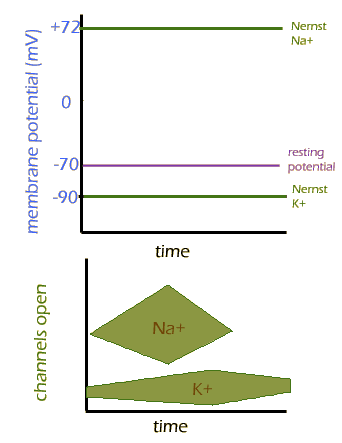
An even (!) easier way to think about the action potential is to use the Nernst equation. Remember each ION has its own Nernst potential. Since the concentration gradients of each don't actually change, all that affects the Nernst equation is their permeabilities. If the membrane is more permeable to sodium, then the membrane potential will be pushed towards the Nernst potential for sodium. Likewise with potassium.
On the graph below, you see the Nernst potential for each ion, and the permeability of the two ions. Try to trace where you expect the membrane potential to be over time, before you mouse over the image:
If you want a printer-friendly version of this module, you can find it here in a Microsoft Word document. This printer-friendly version should be used only to review, as it does not contain any of the interactive material, and only a skeletal version of problems solved in the module.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu