Taking Nernst for a test drive
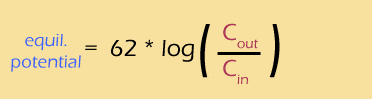

Well, that was a lot of work, but we finally have a way of calculating the equilibrium potential based on the concentration gradient of an ion.
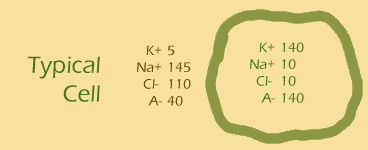 Let's give this equation a run for its money. Remember the typical cell? And remember that the cell's membrane is generally only permeable to potassium (K+)? So...
Let's give this equation a run for its money. Remember the typical cell? And remember that the cell's membrane is generally only permeable to potassium (K+)? So...
What is the Nernst potential associated with the potassium ion?
(To make this problem interactive, turn on javascript!)
- I need a hint: This problem is basically plug-and-chug
- ... another hint ... : put the values of [K+]out and [K+]in into the equation
- ... another hint ... : like this: V = 62 log(5/140)
- ... another hint ... : get your calculator out ! please please please !
I think I have the answer: -90 mV
Bet you thought that was going to be harder... Let's try another one
What is the Nernst potential associated with the sodium ion?
(To make this problem interactive, turn on javascript!)
- plug and chug!: [Na+]out is 145 and [Na+]in is 10
- ... another hint ... : your calculator is your friend!
I think I have the answer: +72 mV
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu