Now you try it...
John and Suzy want to measure the strength of various mixtures of red koolaid. After finding the proper wavelength, they run 10, 20, 40, and 80% solutions through a spec, and get the following data:
concentration | 10% | 20% | 40% | 80% |
|---|---|---|---|---|
absorbance |
.31 OD |
.59 OD |
1.2 OD |
2.3 OD |
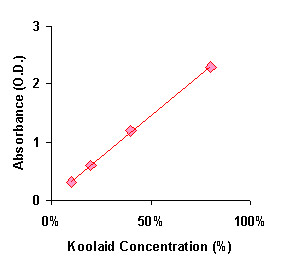

Then they go to the State Fair and get samples of red koolaid from 3 different vendors. These samples have absorbances of 0.7, 1.5, and 2.6 OD. How strong is each glass of koolaid?
First, what is "e"?
(To make this problem interactive, turn on javascript!)
- I need a hint ... : Remember that “e” is the rate at which stuff in the water absorbs light.
- ...another hint ... : Slope = rate, so find the slope of the graph
- ...another hint ... : How much does the absorbance change as you go from 0 to 10% koolaid?
- ...another hint ... : Since Beer's Law says the relationship is DIRECTLY PROPORTIONAL,
then the absorbance will change 1/10 th as much from 0 to 1% as from 0 to 10%.
I think I have the answer: e = 0.03 OD / percent koolaid.
So far, we know that e = 0.03 OD / percent koolaid. Fitting that into our equation, we get
OD = 0.03 c
Now that you have "e", what are the concentrations of the three koolaid samples
(their OD's were 0.7, 1.5, and 2.6 AU's)
(To make this problem interactive, turn on javascript!)
- I need a hint ... : you can write an equation for concentration based on absorbance.
- ...another hint ... : To get the equation for concentration, solve for c...
- ...another hint ... : Solving for c...
OD = e c
c = OD / e
c = OD / 0.03
- ...another hint ... : Use Google as a calculator!
I think I have the answer: 0.7/0.03 = 23%
1.5/0.03=50%
2.6/0.03 = 87%
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu