The dilution factor
When you're thinking about dilution, it helps to simplify your actions into dilution factors. For example, if you
- 1 mL coffee + 4 mL water =
- 1 mL coffee + 9mL water =
- 1 mL + 99mL water =
As you've probably guessed, this works exactly the same whether you're talking about caffeine or meningicocci. Here's what a dilution factor of 0.01 looks like on the lab bench:
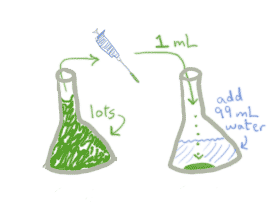
Notice that it really doesn't matter how much of the original stock you started with, as long as you had at least a mL to put into the new container. What matters is how much you transfer and how much water you add. The dilution factor is then defined as
amount transferred / total amount =
amount transferred / (amount transferred + amount water added) =
1 / (1+99) = 1/100 = 0.01.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu