We're going to quickly summarize the module, and then do one final (slightly
extended) example using these equations, and comparing the performance of
the continuous and the discrete models.
 ...................... Review......
...................... Review......
The diffusion through a membrane equations: diffusion is driven by the difference in concentrations on the two sides of the membrane. It is also affected by the permeability coefficient and the area of the membrane:
Continuous version |
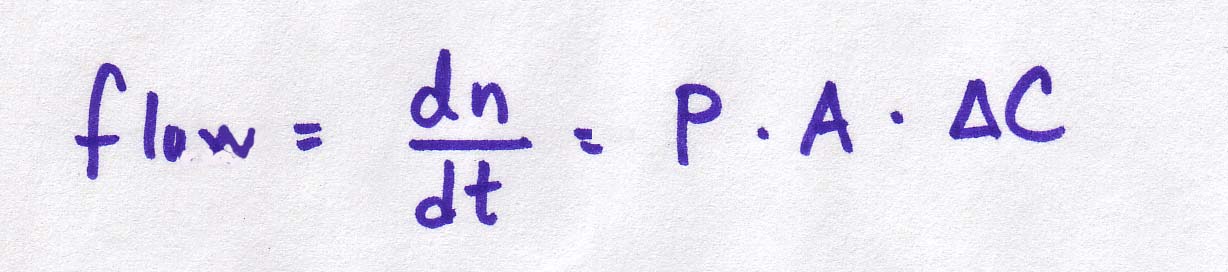
|

|
|---|---|---|
Discrete version |
The permeability coefficient is determined by the equation P = KD/Δx.
The concentrations that we need in order to calculate flux are in moles/liter. A mole is 6.022 * 1023 molecules (sixty trillion trillion).
In order to convert between grams and liters, you need to know the molecular weight of the substance, which is to say, how much a single mole of that substance weighs. Molecular weight is calculated by multiplying the number of times each type of atom appears in the equation by its molecular weight, and adding it all up.
Multiplying grams by molecular weight will give you the number of moles of the substance. Then dividing by the volume of water will give you concentration in moles/L.
Moles per liter is sometimes called simply M.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu