Setting the stage: Diluting Cold Press Coffee
Of course, lots of things can be diluted, not just blood samples...
So let's see what the process of "diluting" looks like.
Here's an everyday example:
 Cold Press coffee is a superstrong form coffee that starts out with about a zillion molecules of caffeine per cup. In order to actually be able to drink the stuff, you have to dilute it.
Cold Press coffee is a superstrong form coffee that starts out with about a zillion molecules of caffeine per cup. In order to actually be able to drink the stuff, you have to dilute it.
For example, you could put one cup of cold press coffee into a pot and add 9 cups of water.
How many molecules of caffeine are in the pot?
How many cups of coffee are in the pot ?
If you pour 1 cup from the pot, how many molecules of caffeine will it have?
Let's look at this in graphical form:
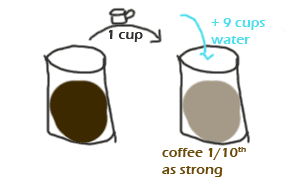
Simple, right? Of course, we don't want to have to draw pictures every time we do a dilution, so we could simply write:
1 cup coffee + 9 cups water --> 1/10th as strong as the original
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu