Putting the "Os" into "Osmolarity"

We need to make one other change. So far we've used the molarity (moles of solute per liter) of solutions like sugar water to as our way of measuring concentration. In truth, the measure of concentration that we need is not molarity, but osmolarity. Osmolarity is is defined by osmoles of solute per liter (kind of like moles, but with an OS on the front). The abbreviation, which we'll use a lot, is OsM.
So what is an osmole? As you may know, when you dissolve salt in water, it disassociates into Na+ and Cl-, in other words, into two pieces. If 1 mole of molecules that dissolves into two "pieces" it creates 2 osmoles. If we started with MgCl2, which dissolves into 3 "pieces", we would get 3 osmoles. Sugar is an organic rather than ionic compound, so it does not disassociate, and 1 mole of sugar dissolves into 1 osmole.
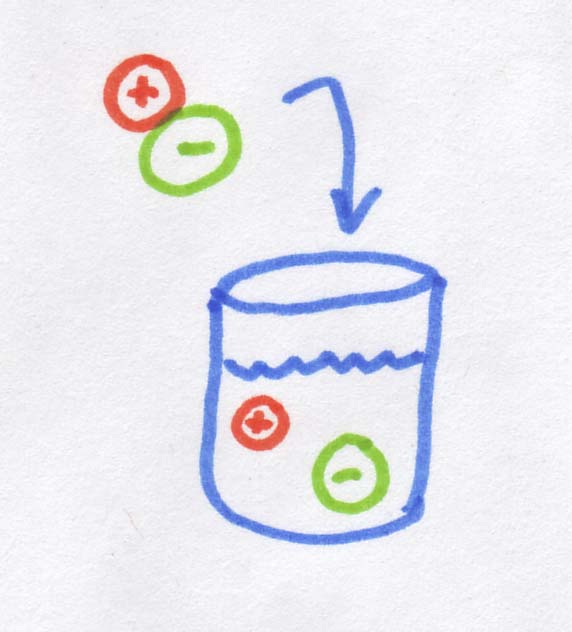
So when we're talking about diffusion across a membrane (including osmosis), it doesn't actually matter that salt ions are smaller than sugar molecules, or that their molecular weight is less, or even that the ions are charged while the sugar molecules are electrically neutral. The only thing that actually matters is how many "pieces" the salt dissociates into. For every salt "molecule" you dump into water, you get TWO ions rather than one, and this is what matters. Essentially a salt molecule provides twice as many "bits" of dissolved stuff as the sugar molecule, so the osmolarity is also doubled.
If I dissolve 2 moles of table salt (NaCl) in 1 litter of water, what osmolarity is produced?
(To make this problem interactive, turn on javascript!)
- I need a hint ... : One molecule of salt makes 2 "bits" when dissolved
- ...another hint ... : 2 moles of salt will make 4 moles of "bits"
I think I have the answer: 4 moles of "bits" / 1L of
water
= 4 OsM
If I dissolve 4 moles of MgCl2 in 2 L of water what will the osmolarity be?
(To make this problem interactive, turn on javascript!)
- I need a hint ... : MgCl2 dissolves into Mg2+ + 2Cl- or 3 bits
- ...another hint ... : osmolarity is "bits" / litters
I think I have the answer: 4 moles MgCl2 = 12 moles of "bits"
12 moles / 2L = 6 OsM
*Yes, I know that salt is an ionic compound and comes in the form of crystalline lattice. However, it is easier to think in terms of a salt "molecule"
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu