Osmosis is a special kind of diffusion
First we told you that diffusion through a membrane was just a special case of plain-vanilla diffusion -- we got to assume that there were only two compartments of interest (left and right), which simplified the math a lot.
 Now,
we're going to tell you that osmosis is a special case
of diffusion through the membrane. And it's the membrane that makes it
special. Recall that membranes vary in terms of their permeability. Some might
be like the proverbial brick wall, while others might be 'leaky as a sieve'.
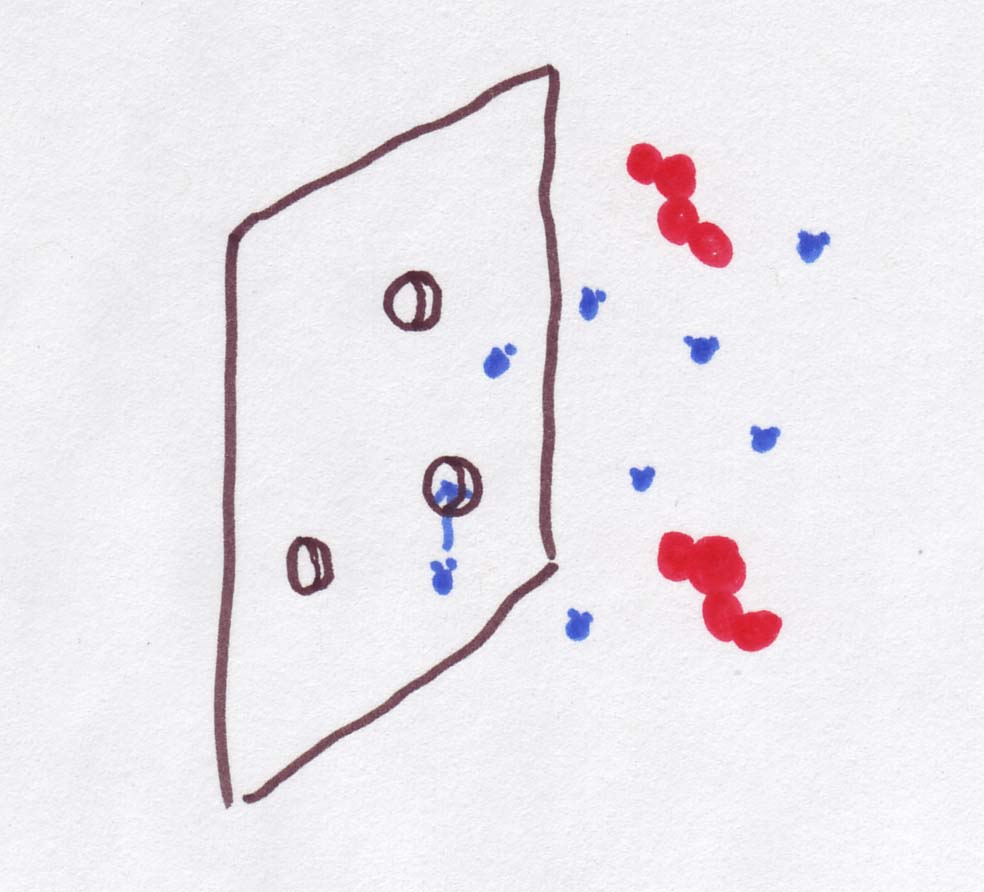
But imagine a membrane with gaps* just big enough to
let water through, but too small to let anything else across.
Now,
we're going to tell you that osmosis is a special case
of diffusion through the membrane. And it's the membrane that makes it
special. Recall that membranes vary in terms of their permeability. Some might
be like the proverbial brick wall, while others might be 'leaky as a sieve'.
But imagine a membrane with gaps* just big enough to
let water through, but too small to let anything else across.
How is this a special case of diffusion through a membrane? Basically, what happens is that water can diffuse but nothing else in the water -- salt, sugar, or other solute -- can get through.
So, instead of general permeability of the membrane, what we care about now is permeability to water, or PH2O .
| Continuous form |  |
|---|
| Discrete form |  |
|---|
*more properly, permeability of the membrane has to do with selective gateways and polarity and so on, but imagining gaps in the membrane will work too.
Clipart for this module thanks to Arthur's Clipart, FreeImages.co.uk, Clipart Heaven
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu