Learning Outcomes
When you have completed this module, you should be able to:
- Define dilution and dilution factor
- Explain why dilutions are necessary for microbial counts
- Prepare dilutions and calculate total dilution of serial dilutions
- Set up an appropriate dilution scheme to result in a countable plate
- Evaluate and interpret the results of plate counts, including selecting the appropriate plate for counting and scaling up from the diluted sample to the original population
Why we need to dilute
For the remainder of this module, we're going to stick with the Viable Plate Count method -- the only method that can tell a live cell from a dead one. But we still have another problem -- the possibility of too many colonies to count. For example, if there are 500 Vibrio per mL and we put 1mL onto a plate, 500 colonies on a Petri dish would look something like this:
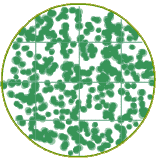
It is so crowded on the plate, that it would not be possible to obtain an accurate count of the number of colonies. And the problem would be even worse if you were trying to count a population in the thousands or millions! You could literally have a carpet of colonies (also known as a 'confluent lawn') growing on your Petri dish. Not only would it be impossible to count the colonies, but not all colony-forming units would be able to form colonies because of the overcrowding and competition for nutrients. This is where dilution saves the day. Not just dilution, but serial dilution... meaning dilution over and over again.
Why do we dilute? To have fewer colonies to count and to obtain a more accurate count.
Why do we do it repeatedly? Because we don't know how much dilution we need. Every time we dilute, we'll also make a new plate to incubate. So we might do 5 dilutions and grow up 5 plates. Then we'll end up throwing away 4 of them. Sound wasteful? Well, dilution and plating is quick and easy compared to the pain of starting your experiment all over again just because your plate has too many or too few colonies.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu