 Putting the "Os" into "Osmolarity"
Putting the "Os" into "Osmolarity"
So far we've used the molarity (moles of solute per litre) of solutions, like sugar water, as our way of measuring concentration. In truth, the measure of concentration that we need is not molarity, but osmolarity. Osmolarity is defined by osmoles of solute per litre (kind of like moles, but with an Os on the front). The abbreviation, which we'll use a lot, is OsM.
So what is an osmole? As you may know, when you dissolve salt in water, it dissociates into Na+ and Cl-, in other words, into two "pieces." If 1 mole of molecules dissolves into two "pieces", it creates 2 osmoles. If we started with 1 mole of MgCl2, which dissolves into 3 "pieces," we would get 3 osmoles. Sugar is an organic rather than ionic compound, so it does not dissociate, and 1 mole of sugar dissolves into 1 osmole. So when you are working out osmolarity the first thing you will need to determine is whether or not the salt in question dissociates into smaller ions.
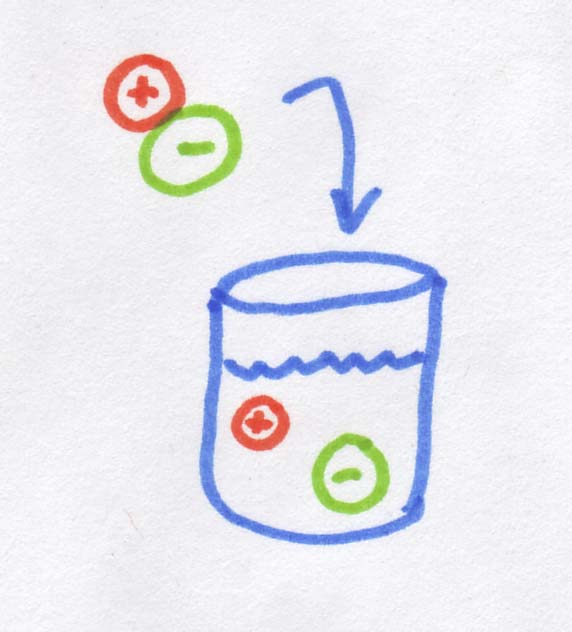
When we're talking about diffusion across a membrane (including osmosis), it doesn't actually matter that salt ions are smaller than sugar molecules, or that their molecular weight is less, or even that the ions are charged while the sugar molecules are electrically neutral. The only thing that actually matters is how many "pieces" the salt dissociates into. For example for a salt "molecule*" that dissociates into two ions, when you dissolve it into water, you will get TWO ions rather than one, and this is what matters for diffusion. Essentially, the salt molecule provides twice as many "bits" of dissolved stuff as the sugar molecule, so the osmolarity is also doubled.
If I dissolve 2 moles of table salt (NaCl) in 1 litre of water, what osmolarity is produced?
(To make this problem interactive, turn on javascript!)
- I need a hint ... : One molecule of salt makes 2 "bits" when dissolved
- ...another hint ... : 2 moles of salt will make 4 moles of "bits"
I think I have the answer: 4 moles of "bits" / 1L of
water
= 4 OsM
If I dissolve 4 moles of MgCl2 in 2 L of water what will the osmolarity be?
(To make this problem interactive, turn on javascript!)
- I need a hint ... : Magnesium chloride dissolves into 2 magnesium and 1 chlorine ion, or 3 bits altogether
- ...another hint ... : osmolarity is "bits" / litres
I think I have the answer: 4 moles MgCl2 = 12 moles of "bits"
12 moles / 2L = 6 OsM
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu