A familiar equation for Fick's First Law
Fick's Law again: Flux is directly proportional to gradient.
Now we know that the gradient is represented by dC/dx, but what does “directly proportional” mean? Put simply, it means this:
small gradient --> small diffusion
BIG gradient --> BIG diffusion
When we have a small gradient, there is not much diffusion going on, but when the gradient is BIG, then diffusion can be substantial. But how can we represent this mathematically? Well, if two quantities are directly proportional, they are related to each other through an equation like y = mx. Hmmm.... this equation should sound familiar -- it is the equation for a line which goes through the origin (0,0).
Continuous version |
Discrete Version |
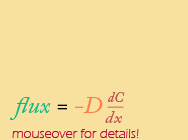 |
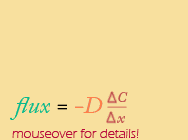 |
So, FINALLY, a mathematical equivalent to Fick's law, in both a continuous (calculus) and discrete version. Notice that they both have the same basic form that is directly analogous to the basic equation for a straight line that intersects the origin (y=mx).
When Fick’s Law is represented on a graph, the flux (like “y”) is plotted along the vertical axis and the gradient (like “x”) is plotted along the horizontal axis. The flux depends (and is therefore called the dependent variable) on two quantities: 1) the steepness of the gradient (in red) and 2) a proportionality coefficient based on the particular substance being measured (called the Diffusion coefficient, "D" - more on that later).
One last note ... Why the negative sign in front of the diffusion coefficient??? This is because diffusion always goes DOWN the concentration gradient -- its direction is opposite to the concentration gradient. That’s why the diffusion coefficient has a negative sign. Movement always occurs in a direction OPPOSITE to that of the gradient.
Review so far...
The Rate of FLOW of particles (dn/dt, i.e. the number of particles moving in a specified time) through an area is the FLUX. The symbol for flux is J and the units of flux are mol m-2 s-1.
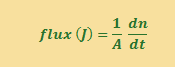
The number of particles n in a certain volume V is the concentration C (C =n/V) so we can also express the flux equation in terms of the change in concentration with time. The units of dC/dt are mol cm-3 s-1.
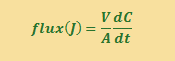
Fick’s First Law states that flux is proportional to the CONCENTRATION GRADIENT, and the proportionality constant D is the DIFFUSION COEFFICIENT.
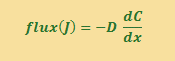
What does the diffusion coefficient, D, really represent biologically? One way to figure that out is to look at the units of D to see what it is really describing. The units of D are length2/time (m2 s-1), and usually reported as cm2/sec (cm2 s-1). The diffusion coefficient (D) describes how long it takes a particular substance (like oxygen or proteins, etc.) to move through a particular medium (like water or molasses).
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu