Visualising the gradient
We saw in the last page that diffusion tends to spread things out, making them less "ordered". A central concept of order then is the “gradient”, a fancy way of saying the difference in particle density between two areas. If there is only a slight difference, then the distribution changes only slightly. But if all the molecules are concentrated on one side, then the distribution changes a lot over time.
Another way to say this is that the NET movement of particles is PROPORTIONAL to the difference between the particle density of two sides – or, proportional to the “steepness” of the gradient.
Which gradient is steeper?
Check your answer by pointing with your mouse.
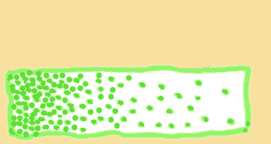 |
or |
 |
So, steeper gradient means faster diffusion.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu