The dilution factor
When you're thinking about dilution, it helps to simplify your actions into dilution factors. When we said the diluted coffee was
"1/10th as strong as the original"
that was a dilution factor. We could also have said
"the dilution factor was 1/10", or
"the dilution factor was 0.1".
Here are a few more for you to try:
- 1 mL coffee + 4 mL water =
- 1 mL coffee + 9mL water =
- 1 mL + 99mL water =
As you've probably guessed, this works exactly the same whether you're talking about caffeine or meningicocci. Here's what a dilution factor of 0.01 looks like on the lab bench:
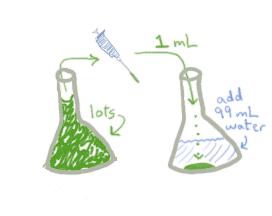
Notice that it really doesn't matter how much of the original stock you started with, as long as you had enough to put 1 mL into the new container. What matters is how much you transfer and how much water you add.
It's possible to write an algebraic expression for the dilution factor, but it's almost more trouble than it's worth, because it sounds so complex. But for what it's worth, the dilution factor for 1 mL stock + 99 mL water is:
amount transferred / total amount =
amount transferred / (amount transferred + amount water added) =
1 / (1+99) = 1/100 = 0.01.
Below is an applet to practice finding dilution factors, and also to determine how much water to add to achieve a given dilution. You should practice this until it is second nature!!

What is the dilution factor?
1 mL stock + mL water
 Can you do it in your sleep? OK, then go on to the next page...
Can you do it in your sleep? OK, then go on to the next page...
photo credits: sleeping student |
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu