Serial Dilution: How it works
The story on the previous pages has many parallels with life in a microbiology lab. Frequently, you will find it necessary to add water (or some other medium) to a stock (or soup, get it?) with a known concentration to make a more dilute solution.
Why would you want to do this? Let's say you need to get 1/10,000th of a mL in order to count the bacteria in it. That would be pretty difficult with a pipette. But instead, you could
- Take that 1 mL and put it in 99 mLs of saline solution. You still have the same number of bacteria, but now they're spread out.
- Now you can take 1 mL of THAT solution, add it to a new container, top off with 99mLs of water, and you'll have only 1/100th of the original bacteria in the mL.
- Pull out 1 mL of this latest mixture, and you'll have 1/100th of 1/100th, which is (multiplying the fractions together) 1/10,000th.
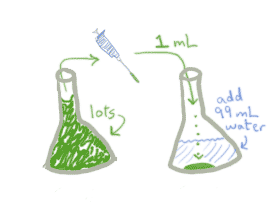
When you do serial dilutions, you multiply together all of the dilution factors. Make sure you are clear on what constitutes a dilution factor. When I add a small amount of the concentrated stuff to an empty container, "top it off" with saline/water/whatever, then remove some small amount, this constitutes a dilution. So above, even though its broken down into 3 steps, there are really only 2 full dilution steps.
When in doubt, try to think it through logically. Often it helps to think through the whole process using some concrete number. For example: "I started with 300,000 cells in a mL, put those into 99 mLs of saline, and took out a mL, so there must have been 3,000 cells in that mL. Then I put those 3,000 cells into 99 mLs of saline, and took out one mL again, so there must have been 30 cells in that mL. Overall my dilution must have been to 30 from 300,000, which is the same as to 1 from 10,000 (1:10,000)." Of course, the idea that you started with 300,000 cells is pure fiction, but it can help you make sure that you've done the dilutions correctly.
Put one mL of a stock into 99 mLs of water. Take 1 mL of that and put it in 999 mLs of water. What is the total dilution?
(To make this problem interactive, turn on javascript!)
- I need a hint ... :Assume that you start with 1,000,000 cells in one mL of solution
- ...another hint ... :After first dilution, you are left with 10,000 cels in one mL of solution
- ...another hint ... :After second dilution, you are left with 10 cells in one mL of solution
- ...another hint ... :Multiply two dilution factors to get the total dilution
I think I have the answer: 1:100,000
Start with a 1:1000 dilution someone else has made. Take 1 mL and put it in 99 mLs of water. What is the total dilution?
(To make this problem interactive, turn on javascript!)
- I need a hint ... :Assume that you start with 10,000 cells in one mL of solution
- ...another hint ... :After first dilution, you are left with 100 cells in one mL of solution
- ...another hint ... :First dilution factor is 1:100
- ...another hint ... :Multiply the dilution factor you obtained with the initial dilution factor to get the total dilution
I think I have the answer: 1:100,000
Put one mL of a stock into 49 mLs of water. Take 1 mL of that and put it in 49 mLs of water. What is the total dilution?
(To make this problem interactive, turn on javascript!)
- I need a hint ... :Assume that you are starting with 20,000 cells in one mL of solution
- ...another hint ... :After first dilution you are left with 400 cells in one mL of solution (20,000/50)
- ...another hint ... :After second dilution you are left with 8 cells in one mL of solution (400/50)
- ...another hint ... :Multiply two dilution factors (1/50 * 1/50) to get the total dilution
I think I have the answer: 1:2500
Put one mL of a stock into 99 mLs of water. Repeat 3 more times. What is the total dilution?
(To make this problem interactive, turn on javascript!)
- I need a hint ... :Assume you are starting with 1,000,000,000 cells in one mL of solution
- ...another hint ... :After first dilution you are left with 10,000,000 cells in one mL of solution (dilution factor 1:100)
- ...another hint ... :Dilution factor is the same each time
- ...another hint ... :Multiply all four dilution factors to get total dilution
I think I have the answer: 1:100,000,000
Start with a 1:10,000 dilution someone else has made. In order to get a 1:100,000 dilution, you need to put 1 mL into __ mLs of water.
(To make this problem interactive, turn on javascript!)
- I need a hint ... :Determine the dilution factor to get 1:100,000 dilution from 1:10,000 dilution
- ...another hint ... :Dilution factor is 1:10
- ...another hint ... :You need to have 10 mL of solution to get 1:100,000 of dilution
I think I have the answer: 9
Starting with a dilution made by a TA, you add 1 mL to 49 mLs of water to get a 1:500,000 dilution. What was the TA's dilution factor?
(To make this problem interactive, turn on javascript!)
- I need a hint ... :Your dilution factor is 1:50
- ...another hint ... :Divide final dilution with your dilution factor to get initial dilution
I think I have the answer: 1:10,000
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu