How fast will osmosis occur?
 |
|---|
We determined which way water flows, but shouldn't it go faster to the right (toward the very concentrated solution) than to the left (a solution that is barely different in concentration)? In fact this is true, and it is directed reflected in the equation for the 2-compartment model:
| Continuous form |  |
|---|
| Discrete form |  |
|---|
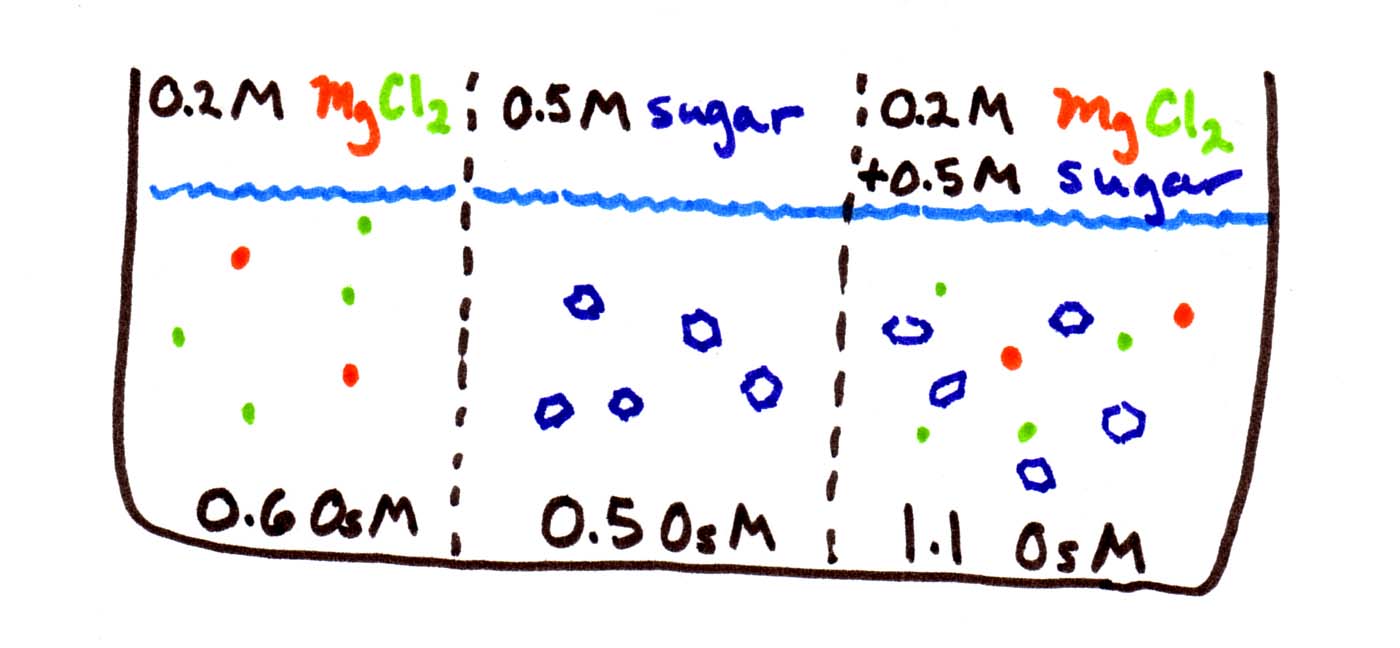
We don't know either the permeability (Pwater) or the area of the membrane (A). But this doesn't really matter if we just want to know how the rates of flow compare. We do know how the concentration differences compare (Cright - Cleft ). Between the first and second compartments, the difference in osmolarity is only 0.1 OsM. Between the second and third compartments, the difference in osmolarity is only 0.6 OsM. So water should flow 6 times faster to the right than to the left.
Of course this state of affairs only lasts for a split second. As soon as water starts to flow, the concentration differences change, and the relative rates of flow change. If you are using the continuous equation, the adjustments will be made immediately. If you are using the discrete version, the adjustment will only be made after Δt time has passed.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu