Beer's Law
Beer's Law explains the relationship between cordial concentration and light
Beer’s Law uses a maths approach to explain the experimentally obtained standard curve. Luckily, the relationship is linear, and all we need to figure out is the slope from the standard curve.
So let's write the equation of a line,

Keeping in mind that ...
- Instead of x, we say C (for concentration).
- Instead of y, we say OD (for optical density, which is the technical way of saying “how much light the cordial absorbed”).
- Instead of m, we say "e" (which stands for “extinction coefficient”, i.e., the amount of light that is extinguished by each extra bit of cordial).
- We know that if there is no cordial in the solution then there is no absorbance so the intercept (b) equals zero
Just to make it a little trickier, the amount of light absorbed also depends is the distance the light has to pass through the cordial solution. So if I had a glass of 10 cm diameter, then the absorbance would be twice that of a glass with 5 cm diameter (if I was looking through the side of the glass). Another linear relationship!
So, to be really correct, we have to write the equation like this:
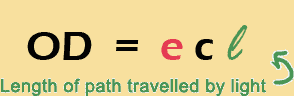
where "l" stands for the “length of the path” that light follows.
Luckily, those people who designed the spectros were pretty smart: they keep the distance that light has to travel constant at 1 cm, which means in practice we can just use the easier equation, OD = ec (that is why the cuvettes have a 1 cm width!) The equation above is known as Beer's Law. Yes, there was a person named Beer (Herr Professor Beer, actually).
In most cases we don’t know the rate at which light gets absorbed for compounds in solution at different concentrations (“e” value or “extinction co-efficient”). We usually experimentally determine the “e” value via a standard curve. This is where the graph bit comes into play!! The slope/gradient or rate of a standard curve is the “e” value.
Are you lost? Lets recap!!
- The “e” value in the equation above is commonly known as the extinction coefficient. We can use it to determine the concentration of a chemical in solution.
- The value of e is the slope/gradient/rate of a line graph of an OD vs concentration graph, i.e., a standard curve.
This is why we make standard curves. The only way to find the correct rate/gradient/slope is by measuring it.
 Oh, now I know why we do so many standard curves in biochemistry!!
Oh, now I know why we do so many standard curves in biochemistry!!
So now we have a second way to determine the cordial concentration using the standard curve.
 Straight Lines/Standard Curves
Straight Lines/Standard Curves