Standard curve–making and using
You need to produce a graph showing the linear relationship between the OD of the solutions (eg cordial) at various concentrations. That means you will get a line graph similar to that shown below (it may flatten out at higher ODs). As the experimenter, you decide what concentrations will be tested and the spectro will measure the ODs of those samples. You should also measure the OD of the unknown (in the case the diluted cordial drink made up by the parents) at the same time. Then plot the OD values for the concentrations you have chosen on a graph (do not use the data on your unknown sample at this point). You need to draw a “line of best fit” through the data points.
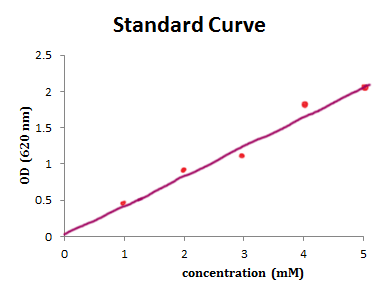
Now, when I say "draw a line of best fit", please DON'T think this means simply connecting the dots as if you were doing a dot-to-dot puzzle. Instead, you want a single straight line that goes approximately though the center of your group of dots (see graph above), so some dots are above and some are below the line, but all are as close as possible. (In statistical jargon, this is known as "doing a linear regression’)
So back to constructing a standard curve for cordial. As we don’t know the concentration of the cordial in molarity, and the manufacturers say you should be using a 20% (v/v) solution of the concentrate, the units of the “x axis” in this case is in %.
You would take a sample of cordial, dilute it to 10% (v/v), read the absorbance using the spectro, and mark your data point on the graph. Then you need to do the same with a 20% (v/v) sample, a 30% (v/v) sample, and a 40% (v/v) sample. Your graph should look something like that shown below.
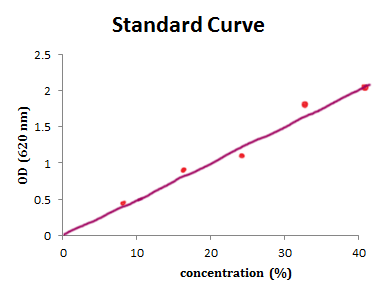
So, for every 20% (v/v) change in concentration, OD increases by about 1.0 unit. The slope of the line is 1.0/20, or 0.05, which is also the value of "e", the extinction coefficient (more on that later).
Using a strandard curve–minimum maths method
So if the kids want to accuse their parents of diluting the cordial too far, they can take their drinks, measure and OD and use the standard curve to determine the concentration of the kids' drinks.
For example, if the OD of the drink were 0.8, you would move along the “y axis” to an OD of 0.8 and then draw a line across to the standard curve, then a perpendicular line and read the value of the “x axis”. You will get the answer approx 15% (v/v) .
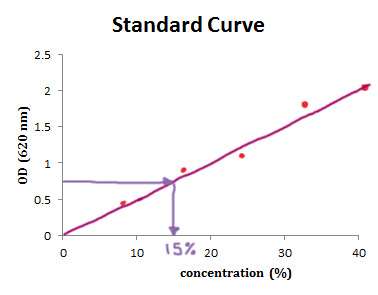
So if you have a spectro in your garage and do the above experiment you now can make a very solid case that your parents are over diluting your drink.
 Straight Lines/Standard Curves
Straight Lines/Standard Curves