It's about "time to diffuse"
Fick’s first law tells us HOW MUCH flux to expect. Fick’s second law gets into more detail, telling us how fast the concentration is changing at any given point in space. This law involves both rates of change with respect to space (position) and time, and takes the form of something called a “partial differential equation”, but we won’t need to go into that much detail here.
If this sounds complicated … it is. We won’t go into how this law works, except to say that when you integrate over time, you get a very useful (and easy to understand) result: a formula for how much time diffusion takes over a specified distance:
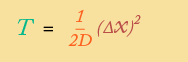 |
 |
Assuming you know a diffusion coefficient, this formula allows you to determine how much time is necessary (on average) for particles moving by diffusion to traverse any given distance.
 Try it out:
Try it out:
If D = 0.00001 cm2/s (for oxygen through water), how long would it take the oxygen to diffuse 0.01 cm below the surface of a still pond?
(To make this problem interactive, turn on javascript!... may not work in Internet Explorer )
- I need a hint ... : Remember: T = (Δx)2 / 2D
- ...another hint ... : x = 0.01 cm!
I think I have the answer: (0.01cm)2 / (2 * 0.00001cm2 / sec)) =
5 sec
How about 0.1 cm?
(To make this problem interactive, turn on javascript!... may not work in Internet Explorer )
I think I have the answer: (0.1cm)2 / (2 * 0.00001cm2 / sec)) =
500 sec
What about 1 cm?
(To make this problem interactive, turn on javascript!... may not work in Internet Explorer )
I think I have the answer: (1cm)2 / (2 * 0.00001cm2 / sec)) =
50,000 sec

Any fish hanging out at 0.1 cm below the water surface would die from lack of oxygen if they had to rely on diffusion to bring them oxygen – – luckily
there are also water currents.
photo credits: pond |
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu